Describe Hydrogen Bonding Between Water Molecules
The polar covalent bond between oxygen and hydrogen creates negative and positive partial charges respectively. 7 How much water is hydrogen and oxygen.
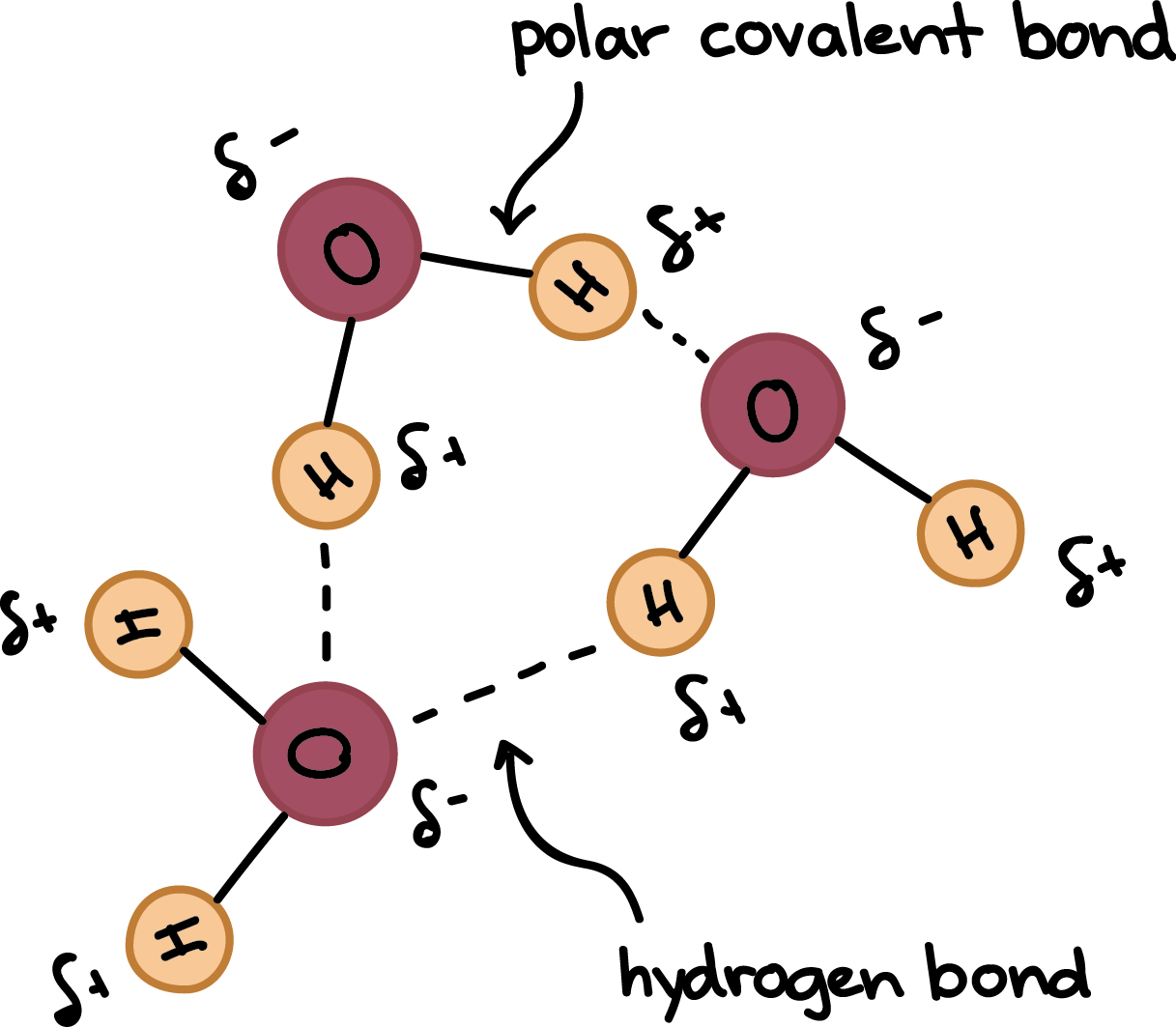
Hydrogen Bonds In Water Article Khan Academy
A water molecule is polar because it has a bent structure and its shared electrons are more attracted to.

. Hydrogen bonds are attractions of electrostatic force caused by the difference in charge between slightly positive hydrogen ions and other slightly negative ions. Explain why hydrogen bonding occurs between water molecules. 6 When hydrogen and oxygen combine to form water water would be.
A The slightly positive hydrogen atom is attracted to the slightly negative oxygen atom within a single water molecule. Water molecules in ice are locked in place apart from. Polarity of water molecules.
1The slightly negative oxygen atom of one water molecule is attracted to the slightly positive hydrogen atom of another water molecule. 1 Describe How Hydrogen And Oxygen Form Water. Now hydrogen bonding can occur due to the electrostatic attraction between the hydrogen atom of one water molecule with δ charge and the oxygen atom of another water molecule with -δ charge.
But the intermolecular bonds the bonds BETWEEN water molecules are the result of hydrogen bonding. Hydrogen bonds between water molecules gives rise to large but mostly compensating energetic changes in enthalpy becoming more negative and entropy becoming less positive. Hydrogen Bonding occurs between 2 adjacent molecules of H 2 O.
In solid water or ice every oxygen atom is stuck bonded to two adjacent hydrogen atoms leading to a regular and predictable crystal lattice pattern of the molecules. - The bond between hydrogen atoms in diatomic hydrogen H2 - Electrons are equally shared between atoms in a covalent bond. Such hydrogen-bonding interaction is VERY STRONG INTERMOLECULAR.
And hydrogen bonding occurs when hydrogen is bound to a strongly electronegative element such as oxygen or nitrogen or fluorine. 1The slightly negative oxygen atom of one water molecule is attracted to the slightly positive hydrogen atom of another water molecule. There is a weak intermolecular force of attraction between the patially positively charged hydrogen atom of.
A hydrogen bond in water occurs between the hydrogen atom of one water molecule and the lone pair of electrons on an oxygen atom of a neighboring water molecule. Hydrogen bonds between water molecules Hydrogen bonds form when a slightly negatively charged part of a molecule comes close to a slightly positively charged hydrogen molecule in the same or another molecule. This happens because the hydrogen atom is attracted to both its own oxygen and other oxygen atoms that come close enough.
8 How do hydrogen bonds form in. - The bond between oxygen and hydrogen in water H2O - Electrons are not equally shared by the atoms in a covalent bond - One atom of a covalent bond has higher electronegativity than the other atom in the bond Nonpolar bonds. Model of hydrogen bonds 1 between molecules of water.
Which of the following statements describe hydrogen bonding between water molecules or describe properties of water that lead to hydrogen bonding between water molecules. They are often described as interactions. This enthalpy-entropy compensation is almost.
Explain how hydrogen bonding occurs between molecules of water. 5 How is water formed. Which of the following statements describe hydrogen bonding between water molecules or describe properties of water that lead to hydrogen bonding between water molecules1The slightly negative oxygen atom of one water molecule is attracted to the slightly positive hydrogen atom of another water molecule2The slightly positive hydrogen atom is.
The attraction between individual water molecules creates a bond known as a hydrogen bond. It is known that oxygen is very electronegative therefore it attracts the electrons creating a polar bond. The adhesion of water is explained by hydrogen bonding of water molecules to other polar surfaces.
The slightly negative oxygen atom of one water molecule is attracted to the slightly positive hydrogen atom of another water molecule. The O-H bond in water is a polar bond. The hydrogen bonds in liquid water restrict the movement of the water molecules so a relatively large amount of water is needed to increase the temperature of water The evaporation of water uses a relatively large amount of energy so water evaporating from the surface removes heat energy from the surface Metabolic Water takes part as a.
3Water is polar due to its bent structure and an unequal sharing of electrons. 2The slightly positive hydrogen atom is attracted to the slightly negative oxygen atom within a single water molecule. A hydrogen bond is an intermolecular attractive force in which a hydrogen atom that is covalently bonded to a small highly electronegative atom is attracted to a lone pair of electrons on an atom in a neighboring.
Hydrogen bonds in water Introduction to the properties of water. This is most easily seen in water Hydrogen bonds are not strong bonds. 1 Water the molecule.
As water switches among the three common states of matter solid liquid and gas the hydrogen bonding that exists between the molecules changes. 2 How is water formed from hydrogen and oxygen. Water molecules have a molecular formula of H2O.
H δ O δ H δ O δ H 2δ O δ H 2. 4 Can water be made with hydrogen and oxygen. A hydrogen bond is a partially electrostatic attraction between a hydrogen H atom which is bound to a more electronegative atom or group such as nitrogen N oxygen O or fluorine Fthe hydrogen bond donorand another adjacent atom bearing a lone pair of electronsthe hydrogen bond acceptor.
The key to understanding waters chemical behavior is its molecular structure. In H 2 O only two of the six outer-shell electrons of oxygen are used for this purpose leaving four electrons which are organized into two non-bonding pairs. Which of the statements describe hydrogen bonding between water molecules or describe properties of water that lead to hydrogen bonding between water molecules.
This happens with all water molecules leaving the oxygen with more electrons and ultimately the more negative side. Thus hydrogen bonds are a very special class of intermolecular attractive forces that arise only in compounds featuring hydrogen atoms bonded to a highly electronegative atom. B A water molecule is polar because it has a bent structure and its shared electrons.
This is because there is a big difference in electronegativity between the oxygen and the water. Both changes are particularly large based by per-mass or per-volume basis due to the small size of the water molecule. 3 What bonds hydrogen and oxygen make water.
The strong dipole of water exerts electrostatic and gravitational forces on charged electrovalent compounds and on the dipoles of polar covalent compounds. And positive and negative forces attract so the now positive hydrogens will bond with. In water each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them.
Hydrogen bonds form between neighboring water molecules when the hydrogen of one atom comes between the oxygen atoms of its own molecule and that of its neighbor. You are a talking tool-making learning bag of water. In the case of water hydrogen bonds form between neighboring hydrogen and oxygen atoms of adjacent water molecules.
The Oxygen in the water molecule will share or the hydrogens lone atom leaving the hydrogen with a more positive charge like in the picture Ive added.

The Strong Polar Bond Between Water Molecules Creates Water Cohesion U S Geological Survey

File 210 Hydrogen Bonds Between Water Molecules 01 Jpg Wikimedia Commons
Belum ada Komentar untuk "Describe Hydrogen Bonding Between Water Molecules"
Posting Komentar